Tryp-N: A Thermostable Protease for the Production of N-terminal Argininyl and Lysinyl Peptides
Abstract
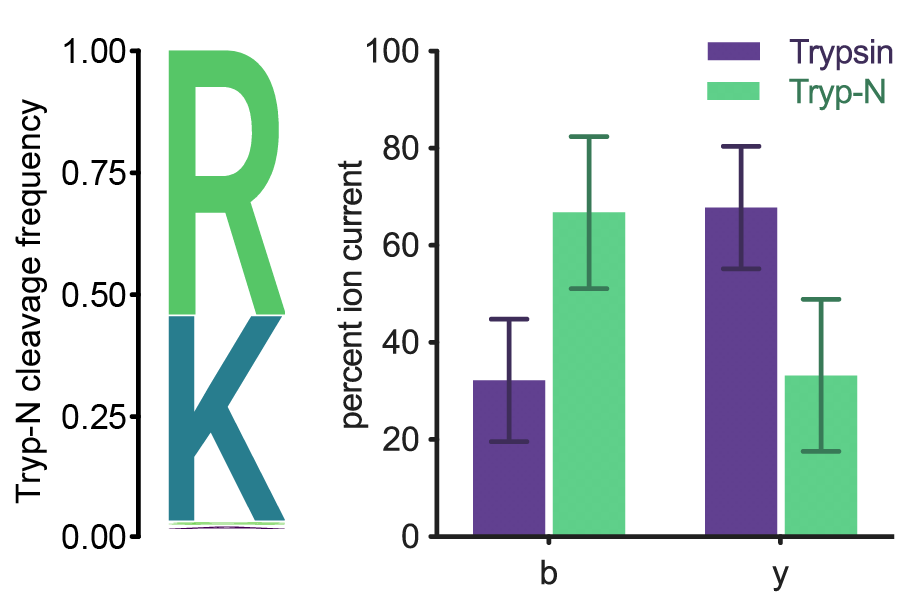 Bottom-up proteomics is a mainstay in protein identification and analysis. These studies typically employ proteolytic treatment of biological samples to generate suitably sized peptides for tandem mass spectrometric (MS) analysis. In MS, fragmentation of peptides is largely driven by charge localization. Consequently, peptides with basic centers exclusively on their N-termini produce mainly b-ions. Thus, it was long ago realized that proteases that yield such peptides would be valuable proteomic tools for achieving simplified peptide fragmentation patterns and peptide assignment. Work by several groups has identified such proteases, however, structural analysis of these suggested that enzymatic optimization was possible. We therefore endeavored to find enzymes that could provide enhanced activity and versatility while maintaining specificity. Using these previously described proteases as informatic search templates, we discovered and then characterized a thermophilic metalloprotease with N-terminal specificity for arginine and lysine. This enzyme, dubbed Tryp-N, affords many advantages including improved thermostability, solvent and detergent tolerance, and rapid digestion time.
Bottom-up proteomics is a mainstay in protein identification and analysis. These studies typically employ proteolytic treatment of biological samples to generate suitably sized peptides for tandem mass spectrometric (MS) analysis. In MS, fragmentation of peptides is largely driven by charge localization. Consequently, peptides with basic centers exclusively on their N-termini produce mainly b-ions. Thus, it was long ago realized that proteases that yield such peptides would be valuable proteomic tools for achieving simplified peptide fragmentation patterns and peptide assignment. Work by several groups has identified such proteases, however, structural analysis of these suggested that enzymatic optimization was possible. We therefore endeavored to find enzymes that could provide enhanced activity and versatility while maintaining specificity. Using these previously described proteases as informatic search templates, we discovered and then characterized a thermophilic metalloprotease with N-terminal specificity for arginine and lysine. This enzyme, dubbed Tryp-N, affords many advantages including improved thermostability, solvent and detergent tolerance, and rapid digestion time.